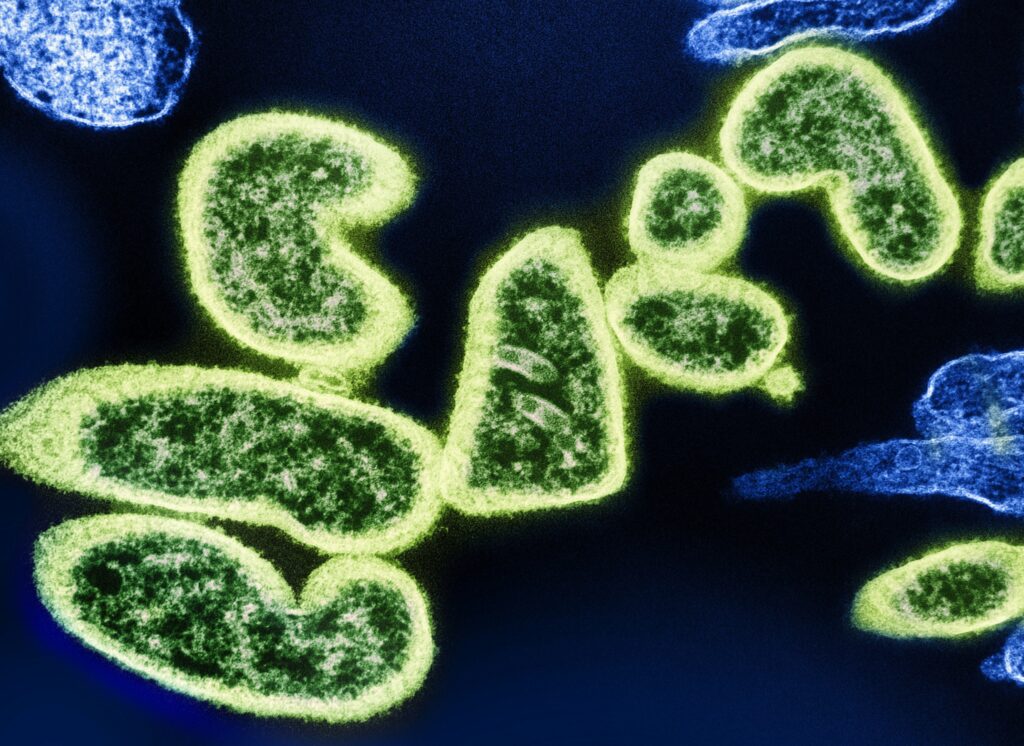
Antibacterial resistance is what happens when bacteria evolve to survive drugs that were meant to kill them. These are the so-called ‘superbugs’ — not science fiction, just science catching up with misuse. They’re stubborn, dangerous, and they’re spreading.
How do they get this way? Overprescription is a big one. Doctors handing out antibiotics for viral infections — which antibiotics can’t treat — just trains bacteria to adapt. Then there’s misuse. People not finishing their antibiotic courses or self-medicating finish the job of creating these resistant strains. And outside the clinic, antibiotics are pumped into livestock feed to keep farms running smoothly. That adds up.
The result is a slow-building crisis. Infections once cured in days now linger or even become deadly. The options for treatment are shrinking. We risk rolling back decades of medical progress if we keep dosing recklessly. The bugs aren’t getting smarter — we’re just giving them too many chances to survive.
Drug-resistant bacteria aren’t science fiction anymore. They’re here and spreading. Some of the most notorious strains today include MRSA (methicillin-resistant Staphylococcus aureus), CRE (carbapenem-resistant Enterobacteriaceae), and drug-resistant TB. These bugs don’t just sit around in labs. They’re out in the world and evolving fast.
Take CRE, for instance. It’s turning up in hospitals across the U.S. and causing infections that can be almost impossible to treat. In some outbreaks, the death rate has reached 50 percent. In other parts of the world, like India and Brazil, resistant strains of E. coli and Klebsiella are tearing through communities, making routine infections dangerous again.
Hospitals and clinics are hot zones. You’ve got high antibiotic use, vulnerable patients, and constant movement. It’s the perfect breeding ground. Even with sterilization protocols, superbugs find cracks to crawl through. Vents, IV lines, even hands that weren’t scrubbed long enough. Once they’re in, they spread fast.
Bottom line: Antibiotic resistance isn’t coming. It’s already happening. And if the places meant to heal us are turning into launchpads for superbugs, it’s a problem that can’t be shrugged off.
Antimicrobial resistance isn’t just something that happens in hospitals or labs. Much of it starts in everyday choices. People take antibiotics for viral infections, skip full courses of meds, or use leftover prescriptions. In livestock, antibiotics are often used to speed up growth, not cure sickness. These habits quietly train bacteria to survive, evolve, and fight back.
Combine that with modern travel patterns and the sheer speed of global movement, and resistant strains can circle the planet in days. One person’s misuse in one country can ripple to another. Add in climate change—warmer temperatures, shifting habitats, denser urban zones—and you get new environments where infections spread faster and more broadly.
Making things worse? You can carry a resistant infection without ever showing symptoms. These quiet carriers move between countries, homes, and public spaces, unaware they’re fueling the spread. Resistance doesn’t always knock loudly. Often, it moves in silence—until it’s too late.
Antibiotics still offer a lifeline, but the list of effective options is getting shorter. Drugs like vancomycin, daptomycin, and a few advanced cephalosporins are holding the line—for now. These medications continue to work against many resistant strains, but they’re expensive, require careful dosing, and lose effectiveness over time.
The real problem is the pipeline. Developing a new antibiotic is slow, costly, and often unrewarding for pharmaceutical companies. Most big players have stepped back, leaving smaller firms and academic labs to take the lead. The result? Fewer new drugs, and a dangerous reliance on decades-old treatments.
Meanwhile, bacteria aren’t waiting. Researchers are in a race against microbial evolution, trying to stay one step ahead through combination therapies, synthetic biology approaches, and new delivery systems. But none of this moves fast.
That’s why infection control is more important than ever. Hospitals are doubling down on hygiene training. Antibiotic stewardship campaigns—programs to use drugs more wisely—are gaining traction. And simple measures like hand-washing, controlled antibiotic prescriptions, and rapid diagnostics are saving real lives even before treatment begins.
In the absence of a miracle cure, smart prevention and cautious prescribing are what keep things from breaking down.
Bacteriophage therapy isn’t science fiction anymore. It’s real, it’s precise, and it’s giving antibiotics a run for their money. By leveraging viruses that specifically target harmful bacteria, clinicians can treat infections in a way that’s less blunt-force and more sniper-mode. What’s new in 2024 is how genomic targeting is making this even smarter. We’re talking about matching phages to bugs based on DNA—not guesswork.
That’s where AI comes in. Systems are now being trained to map bacterial genomes and predict how they might evolve resistance. Instead of playing catch-up after a drug stops working, software models are getting ahead of the curve. They simulate mutations, evaluate structural shifts, and suggest next-gen phage matches before bacteria even get the upper hand.
It’s early days, but the combo of custom phage design plus AI foresight could reshape everything from hospital treatment protocols to how we fight superbugs globally.
(Further reading: AI in Medicine – How It’s Shaping Health and Wellness)
Smart Antibiotic Habits for Individuals and Families
Most people don’t need antibiotics as often as they think. A sore throat doesn’t always mean strep. A cough isn’t always bacterial. One of the smartest health choices individuals and families can make is learning when to say no to a prescription. If your doctor says it’s a virus, trust the diagnosis. Antibiotics won’t speed up recovery, and misusing them builds resistance—both in your body and in society at large.
At home and in workplaces, cleanliness is key but overuse of antibacterial products isn’t helping. Regular soap and water does the job in most situations. Bleaching every surface every day? Not necessary. In fact, excessive disinfecting can contribute to the problem by wiping out good bacteria and encouraging the tough ones to thrive. Focus on routine hand washing, keeping shared items clean, and minimizing unnecessary antibiotic-based cleaners.
If you’ve got kids, teach them early: medicine is powerful, but more isn’t always better. Saving antibiotics for when they’re absolutely necessary makes them more likely to work when you really need them. It’s not about ignoring health—it’s about using our tools wisely.
Governments around the globe are slowly waking up to the threat of antimicrobial resistance (AMR). Policy shifts are becoming more common, with national action plans emerging in countries once considered passive players. Some nations are putting strict regulations on antibiotic use in agriculture, cracking down on overprescription, and funneling more funding into research. Still, progress is uneven, and enforcement remains a challenge.
Hospitals are building smarter resistance tracking systems. They’re using data analytics to monitor outbreaks, detect resistance patterns early, and respond in real time. Some healthcare networks have integrated centralized dashboards that allow facilities to share information quickly. The aim is clear: reduce spread, improve accountability, catch threats before they go systemic.
Public awareness campaigns are also picking up steam. While the message doesn’t always land on the first try, more people are beginning to understand that AMR isn’t just a hospital problem — it’s a community problem. Local health departments, global NGOs, and even influencers are putting out digestible content on when to take antibiotics, how resistance develops, and why every small action matters. The campaigns that actually work? The ones that keep it simple, specific, and backed by trusted voices.
The threat isn’t hype. It’s real, measurable, and already changing how digital platforms function. Whether it’s an algorithm shift, rising AI content floods, or the race toward ultra-short attention spans, the rules are being rewritten. Sitting this one out isn’t an option.
The cost of doing nothing? Irrelevance. Dropping views. Burnout from chasing trends blindly. Creators who ignore early signals may find themselves locked out of visibility loops or struggling to rebuild trust with their audience. On the flip side, those who read the signals and adapt quickly gain an edge. They spend less time reacting and more time building meaningful content that lands.
This isn’t a fight we watch from the sidelines. It’s something we can shape—if we act early and stay consistent. That means tracking updates, testing new formats, and using the tools that help you move faster. If creators get ahead of these shifts instead of chasing them, 2024 becomes an opportunity, not a warning.